Abstract
Background Richter transformation (RT) of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) is a known but uncommon complication with dismal prognosis despite improvements in CLL outcomes with novel agents. Previously published studies are characterized by cohort heterogeneity with respect to CLL treatments (predating the rituximab era and novel agents) and RT histology [diffuse large B-cell lymphoma (DLBCL) and classic Hodgkin lymphoma (cHL)], limiting their generalizability in the modern era. To this end, we examined characteristics and outcomes in 222 DLBCL-RT cases, enriching for patients treated with novel agents.
Methods We collected 247 RT cases diagnosed between 1995-2022 across eight academic institutions, excluding 23 with cHL-RT and 2 with "pseudo-transformation" after BTKi withdrawal (final n = 222; 41% of cases post-2010). We analyzed > 65 CLL/RT biologic, pathologic, and clinical parameters. Primary outcomes included complete response (CR) to first line of therapy [in CLL (iwCLL 2008/2018) and RT (Lugano 2014)] and 5-year overall survival (5yr-OS) after RT, estimated using Cox proportional hazards [yielding hazard ratios (HRs) and 95% confidence intervals (CIs)] and flexible parametric survival models (for prediction of standardized survival).
Results The antecedent CLL included: 11% cases with atypical CLL, 84% IGHVUM, and 70% ZAP70+ with 46% and 43% of patients harboring del(13q) and del(17p), respectively. CLL/RT was synchronous (CLL diagnosis within 2 months of RT diagnosis) in 13% of cases. Only del(17p) correlated with C/HC (≥ 3 / ≥ 5 chromosome abnormalities) karyotype (p < 0.0001), and ZAP70/CD38 had no association with IGHV status. RT was primarily nodal (47.3%), non-GCB (90.3%), and EBV-negative (95%) with p53 IHC-positive large cells in 60% of cases and MYC translocation in 10%.
Preceding CLL was treated with a median of 2 lines of therapy (range: 1-7) with 53% heavily-treated (≥ 2 lines). The most common first lines (LOT1) were chemotherapy (fludarabine-based or bendamustine/CHOP equivalents; 73.6%) and BTKi/novel agents (17%); 37% received BTKi as any LOT, while 44% received any novel agent (BTKi, BCL2i, PI3Ki, or SYKi). CLL CRLOT1 was achieved in 41.2% with overall response of 77%. Median time to RT from CLL diagnosis (sequential group) was 47.6 months pre-2010 (IQR: 87.8) vs. 21.8 months post-2010 (IQR: 34.6, p < 0.0001).
During a median follow up of 10.2 months (IQR: 27.1 months) post-RT, 59% of patients died. Median OS was 17.4 months (range: 11.3-24.2) with 28% 5yr-OS. In the pre-rituximab era (i.e. prior to 2010), median OS was significantly shorter compared to the present era (8 vs. 19.9 months). Indicators of inferior 5yr-OS on univariable analysis included: sequential transformation (HR: 2.34 [95% CI: 1.25-4.35]), not achieving CRLOT1 for CLL (HR: 1.62 [95% CI: 1.01-2.58]), heavily-treated CLL (HR: 1.98 [95% CI: 1.36-2.88]), and C/HC karyotype (HR: 1.59 [95% CI: 1.02-2.46]). While trisomy 12 predicted superior outcome (HR: 0.54 [95% CI: 0.32-0.94]), del(13q) and del(17p) had no impact.
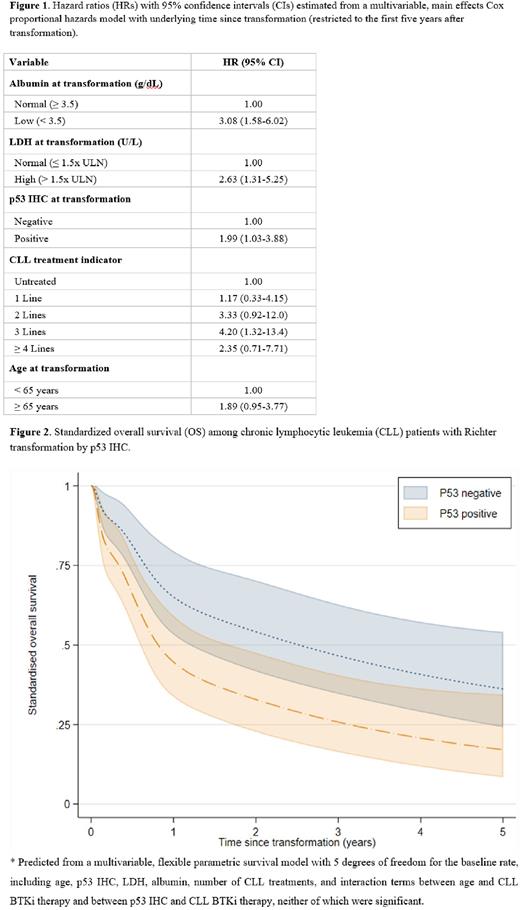
Adverse baseline covariates at RT diagnosis were low (< 3.5 g/dL) serum albumin (HR: 3.13 [95% CI: 2.09-4.69]), low (< 150x103/uL) platelet count (HR: 2.38 [95% CI: 1.64-3.47]), LDH > 1.5x ULN (HR: 1.63 [95% CI: 1.12-1.38]), and ECOG-PS ≥ 2 (HR: 2.4 [95% CI: 1.51-3.81]). Notably, p53 IHC positivity in RT predicted increased all-cause mortality (HR: 2.13 [95% CI: 1.27-3.5]), retaining significance in both minimally- and heavily-treated CLL subsets. RT was treated using CHOP-R or EPOCH-R in 62%. Not achieving CRLOT1 for RT predicted worse 5yr-OS (HR: 3.90 [95% CI: 2.09-7.29]). A multivariable, main effects Cox model was constructed using five variables (age at transformation, CLL treatment number, LDH > 1.5x ULN, albumin, and p53 IHC). In this age-adjusted model, all variables retained adverse prognostic significance (Figure 1). Marginal OS was predicted by p53 IHC, standardizing over remaining covariates, and with a trend towards inferior 5yr-OS in p53-positive vs. -negative RT (13% vs. 38%; Figure 2).
Conclusion This multicenter, international study is the largest cohort of RT in the era of novel therapies with solely DLBCL histology. We confirmed the utility of several traditional predictors. In addition, we identified p53 IHC as a promising predictor of adverse outcome (in univariable and multivariable analyses) based on these preliminary analyses.
Disclosures
Riedell:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Nektar Therapeutics: Membership on an entity's Board of Directors or advisory committees; Intellia Therapeutics: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Sana Biotechnology: Consultancy; AbbVie: Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy; Takeda: Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite/Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; MorphoSys: Research Funding; Calibr: Research Funding; Tessa Therapeutics: Research Funding; Fate Therapeutics: Research Funding; Xencor: Research Funding. Thirman:AbbVie,Gilead Sciences,Janssen,Merck,Pharmacyclics, Syndax, TG Therapeutics, Tolero Pharmaceuticals.: Consultancy, Research Funding; AbbVie, AstraZeneca, Celgene,Janssen, Pharmacyclics, Roche/Genentech: Consultancy. Patel:Celgene/BMS: Research Funding; Pfizer: Research Funding; Servier/Agios: Research Funding; Kronos Bio: Research Funding; AbbVie: Consultancy. Bishop:Bristol Myers Squibb: Honoraria, Other: Travel support, Speakers Bureau; Novartis: Consultancy, Honoraria, Other: Travel support , Research Funding; CRISPR Therapeutics: Consultancy, Research Funding; Agios: Consultancy, Honoraria, Other: Travel support, Speakers Bureau; Iovance: Consultancy; Bluebird Bio: Consultancy; WindMIL Therapeutics: Consultancy; Arcellx: Consultancy, Research Funding; Autolus: Consultancy, Research Funding; Immatics: Research Funding; Triumvira: Research Funding; Tmunity: Research Funding; Chimeric Therapeutics: Consultancy; Sanofi: Honoraria, Speakers Bureau; Sana Biotechnology: Consultancy; Servier: Speakers Bureau; ADC Therapeutics: Speakers Bureau; Celgene: Honoraria; Incyte: Honoraria, Other: Travel support , Speakers Bureau; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel support, Research Funding, Speakers Bureau. Behdad:Leica: Honoraria; Lilly: Honoraria; advisory committee: Membership on an entity's Board of Directors or advisory committees; Thermofisher scientific: Honoraria; Leica: Membership on an entity's Board of Directors or advisory committees; Foundation Medicine/Roche China: Honoraria. Larson:Servier: Consultancy; Novartis: Consultancy, Research Funding; MorphoSys: Consultancy; MedPace: Consultancy; CVS/caremark: Consultancy; BMS: Consultancy; Immunogen: Consultancy; Astellas: Consultancy, Research Funding; Takeda: Consultancy; Epizyme: Consultancy; Amgen: Consultancy; Daiichi Sankyo: Research Funding; Rafael Pharmaceuticals: Research Funding; Gilead: Research Funding; Celgene: Research Funding; cellectis: Research Funding. Smith:Kite Pharma: Consultancy; Genentech: Consultancy; Bayer: Consultancy; TGTX: Consultancy; ADC Therapeutics: Consultancy; Gilead: Consultancy; BMS: Consultancy; Morphosys: Consultancy; Adaptive: Consultancy; Janssen: Consultancy; Karyopharm: Consultancy; Celgene: Consultancy; Portola: Research Funding; Gamida Cell: Consultancy; Bantam: Consultancy; Karyopharm: Consultancy; Chair, Lymphoma Research Foundation SAB: Membership on an entity's Board of Directors or advisory committees. Kline:iTeos, Merck, Verastem: Research Funding; Karyopharm, Kite/Gilead, Merck, MorphoSys, Seagen, Verastem: Consultancy. Ku:Genor BioPharma: Consultancy; Antengene: Consultancy; Roche: Consultancy; St Vincent's Hospital: Current Employment. Venkataraman:American society of Hematology: Honoraria; EUSA Pharma: Speakers Bureau; Wolters-Kluwer: Patents & Royalties: Royalty for editor of a book, $1000 for 2021.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal